;)
Addressing the Global Burden of Antimicrobial Resistance
The increasing burden of AMR and significant decline in the development and commercialization of new antibiotics has created a global health crisis associated with significant mortality.
There is an urgent need for new and differentiated classes of antibiotics, with clinical utility, that successfully treat infections and overcome the combined challenges of intrinsic resistance and cross-resistance to current classes of marketed agents.
Antibiotic Resistance Is a Growing and Global Health Crisis
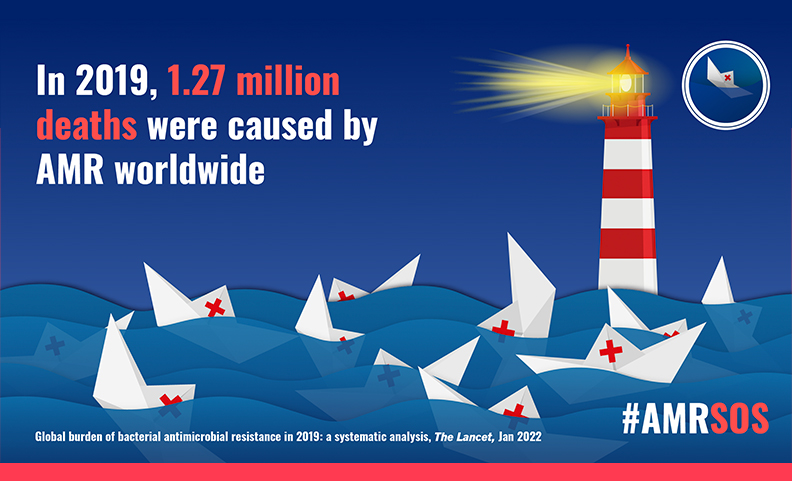
The Centers for Disease Control and Prevention has reported that more than 2.8 million antibiotic-resistant infections occur in the U.S. each year, and more than 35,000 people die as a result*. According to analysis of data from 2019 (The Lancet GRAM Report, 2022), at a global scale, 1.27 million deaths could be directly attributed to drug resistant infections**. It is also estimated that the annual cost to the U.S. healthcare system is between $21 billion and $34 billion. Based on scenarios of rising drug resistance for six pathogens to 2050, the UK-commissioned ‘O’Neill Report’ estimated that, ‘unless action is taken, the burden of deaths from AMR could balloon to 10 million lives each year by 2050, at a cumulative cost to global economic output of $100 trillion’***.
For patients, drug resistant infections can potentially turn a routine procedure into an extended hospital stay and potential life-threatening situation as physicians run through the current, often inadequate, antibiotic armamentarium.
Without the discovery and commercialization of new antibiotics, the success of modern medicine in treating serious and life-threatening diseases is at significant risk.
*Source: 2019 Antibiotic Resistance Threats Report | CDC, **Source: GRAM Report, ***Source: O’Neill Report
“The clinical pipeline and recently approved antibiotics are insufficient to tackle the challenge of increasing emergence and spread of antimicrobial resistance.”
The Pipeline of New Antibiotics Is Running Dry
Discovering and developing new classes of antibiotics is extremely important. Many new and recently approved antibiotics are based on drugs from existing classes targeting only a limited number of mechanisms of action and thereby rendering them susceptible to the same resistance mechanisms as their parent drugs (known as ‘cross-resistance’).
More than 80% (9 / 11) of antibiotics approved by FDA or EMA from 2017-2020 were “from existing classes where resistance mechanisms are well established and rapid emergence of resistance is foreseen.” Of the 26 clinical stage antibiotics, only seven fulfil at least one of the four WHO innovation criteria*.
The challenge is particularly acute for Gram negative infections, and it has been more than 30 years since the U.S. approval of a Gram-negative antibiotic with a novel mechanism of action. There is a long-standing concern amongst scientists, doctors, public health officials, and other stakeholders regarding the dangerously low number of antibiotics in development to address current and future patient needs, particularly for treating the most urgent bacterial threats, such as Gram-negative pathogens, and those prioritized by the CDC and WHO**.
Targeting unexploited and under-exploited antibiotic mechanisms is an important approach to deliver antibiotics with durable clinical utility. ArrePath’s strategy and platform identifies compounds that target such unexploited mechanisms and also optimizes them to overcome intrinsic resistance and deliver new classes of antibiotics.
*Source: WHO, **Source: PEW Charitable Trusts